1. 調査手法および範囲
1.1. 調査手法
1.2. 調査目的および報告書の範囲
2. 定義および概要
3. エグゼクティブサマリー
3.1. 治療の種類別抜粋
3.2. 用途別抜粋
3.3. 地域別抜粋
4. 動向
4.1. 影響因子
4.1.1. 推進要因
4.1.1.1. 高脂血症および心血管疾患(CVD)の有病率上昇
4.1.1.2. XX
4.1.2. 阻害要因
4.1.2.1. 限られた治療オプションの可用性
4.1.3. 機会
4.1.4. 影響分析
5. 産業分析
5.1. ポーターのファイブフォース分析
5.2. サプライチェーン分析
5.3. 価格分析
5.4. 規制分析
6. 治療の種類別
6.1. はじめに
6.1.1. 治療の種類別分析および前年比成長率(%)
6.1.2. 治療の種類別市場魅力度指数
6.2. モノクローナル抗体 *
6.2.1. はじめに
6.2.2. 市場規模分析および前年比成長率分析(%)
6.3. アンチセンスオリゴヌクレオチド(ASO)
6.4. RNA干渉(RNAi)療法(siRNA)
6.5. CRISPR ベースの遺伝子編集
6.6. その他
7. 用途別
7.1. はじめに
7.1.1. 用途別市場規模および前年比成長率(%)
7.1.2. 用途別市場魅力度指数
7.2. 家族性高コレステロール血症(FH)*
7.2.1. はじめに
7.2.2. 市場規模分析および前年比成長率分析(%)
7.3. 難治性高脂血症
7.4. 混合型脂質異常症
7.5. 心血管疾患
7.6. その他
8. 地域別
8.1. はじめに
8.1.1. 地域別市場規模分析および前年比成長率分析(%)
8.1.2. 市場魅力度指数、地域別
8.2. 北米
8.2.1. はじめに
8.2.2. 主な地域特有の動向
8.2.3. 市場規模分析および前年比成長率分析(%)、治療の種類別
8.2.4. 市場規模分析および前年比成長率分析(%)、用途別
8.2.5. 国別の市場規模分析および前年比成長率分析(%)
8.2.5.1. 米国
8.2.5.2. カナダ
8.2.5.3. メキシコ
8.3. ヨーロッパ
8.3.1. はじめに
8.3.2. 地域別の主な動向
8.3.3. 治療の種類別市場規模分析および前年比成長率(%)
8.3.4. 用途別市場規模分析および前年比成長率(%)
8.3.5. 国別市場規模分析および前年比成長率(%)
8.3.5.1. ドイツ
8.3.5.2. 英国
8.3.5.3. フランス
8.3.5.4. スペイン
8.3.5.5. イタリア
8.3.5.6. ヨーロッパのその他地域
8.4. 南アメリカ
8.4.1. はじめに
8.4.2. 地域特有の主な動向
8.4.3. 市場規模分析および前年比成長率分析(%)、治療の種類別
8.4.4. 用途別市場規模分析および前年比成長率(%)
8.4.5. 国別市場規模分析および前年比成長率(%)
8.4.5.1. ブラジル
8.4.5.2. アルゼンチン
8.4.5.3. 南米その他
8.5. アジア太平洋地域
8.5.1. はじめに
8.5.2. 地域別主要動向
8.5.3. 市場規模分析および前年比成長率分析(%)、治療の種類別
8.5.4. 市場規模分析および前年比成長率分析(%)、用途別
8.5.5. 市場規模分析および前年比成長率分析(%)、国別
8.5.5.1. 中国
8.5.5.2. インド
8.5.5.3. 日本
8.5.5.4. 韓国
8.5.5.5. アジア太平洋地域その他
8.6. 中東およびアフリカ
8.6.1. はじめに
8.6.2. 主要地域特有の動向
8.6.3. 治療の種類別市場規模分析および前年比成長率(%)
8.6.4. 用途別市場規模分析および前年比成長率(%)
9. 競合状況
9.1. 競合シナリオ
9.2. 市場ポジショニング/シェア分析
9.3. 合併・買収分析
10. 企業プロフィール
Regeneron Pharmaceuticals, Inc.
Arrowhead Pharmaceuticals Inc.
Amgen Inc.
Ionis Pharmaceuticals, Inc.(Akcea Therapeutics)
Silence Therapeutics
Eli Lilly and Company
Novartis AG
Verve Therapeutics, Inc.
CRISPR Therapeutics
リストは網羅的なものではありません。
11. 付録
11.1. 当社およびサービスについて
11.2. お問い合わせ
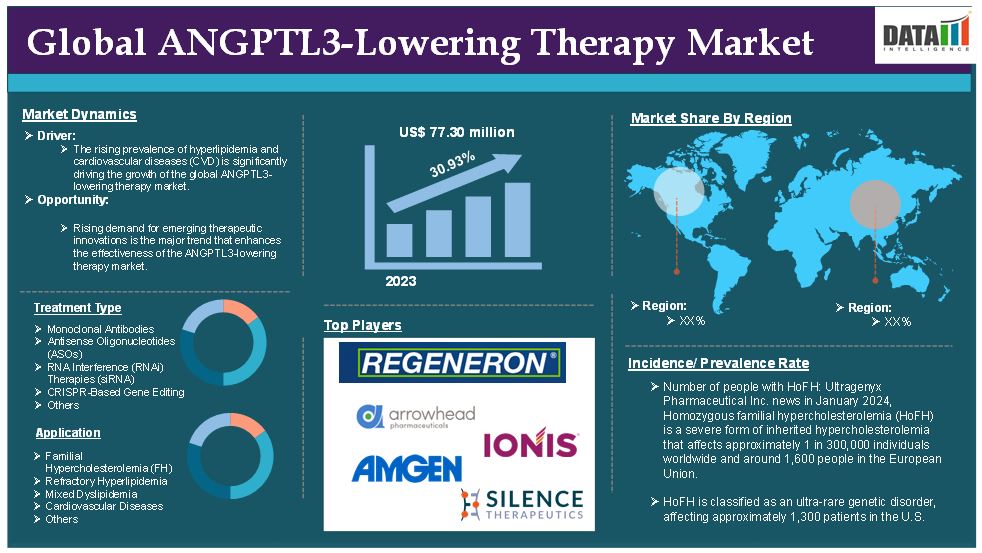
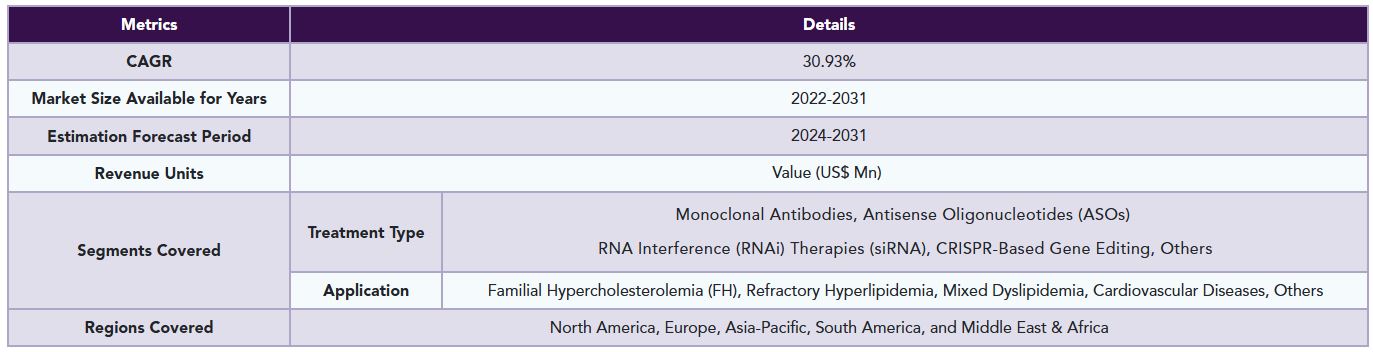
The global ANGPTL3-lowering therapy market reached US$ 77.30 million in 2023 and is expected to reach US$ 785.21 million by 2031, growing at a CAGR of 30.93% during the forecast period 2024-2031.
ANGPTL3-lowering therapy consists of various pharmacological agents aimed at inhibiting or decreasing the activity of ANGPTL3. This action facilitates the clearance of triglyceride-rich lipoproteins and results in lower levels of low-density lipoprotein cholesterol (LDL-C). These therapies are especially important for patients with conditions like homozygous familial hypercholesterolemia (HoFH) and other types of dyslipidemia, where conventional lipid-lowering treatments may not be adequate.
The ANGPTL3 protein is a glycoprotein produced by liver cells that plays a crucial role in lipid metabolism alongside other ANGPTL proteins. Its inhibition represents a promising strategy to further lower LDL-C levels and mitigate cardiovascular risk in patients who do not respond adequately to traditional therapies. The pharmacological inhibition of ANGPTL3-lowering therapy using the monoclonal antibody evinacumab has demonstrated a 50% reduction in LDL-C, even in patients with homozygous familial hypercholesterolemia (HoFH). This approach holds considerable promise for further reducing ASCVD risk. These factors have driven the global ANGPTL3-lowering therapy market expansion.
Executive Summary
Market Dynamics: Drivers & Restraints
Rising prevalence of hyperlipidemia and cardiovascular diseases (CVD)
The rising prevalence of hyperlipidemia and cardiovascular diseases (CVD) is significantly driving the growth of the global ANGPTL3-lowering therapy market and is expected to drive throughout the market forecast period.
The rising prevalence of hyperlipidemia, a major contributor to cardiovascular diseases (CVD), is fueling the demand for effective lipid-lowering treatments, particularly those that target the ANGPTL3-lowering therapy. Inhibitors of ANGPTL3 have emerged as a promising therapeutic option for controlling lipid levels, especially in patients who do not adequately respond to conventional therapies. These inhibitors are particularly beneficial for individuals with difficult-to-manage hyperlipidemia, offering an alternative approach to lipid management that addresses unmet clinical needs in this patient population.
Cardiovascular diseases & hyperlipidemia remain the leading cause of death worldwide, significantly linked to elevated levels of low-density lipoprotein cholesterol (LDL-C) and triglycerides. The growing incidence of CVD fuels the need for effective lipid-lowering therapies, including those targeting ANGPTL3-lowering therapy.
Cardiovascular disease (CVD) & hyperlipidemia stand as the leading cause of mortality worldwide, heavily influenced by elevated plasma levels of low-density lipoprotein cholesterol (LDL-C) and the resultant formation of atherosclerotic plaques. Physicians have access to various LDL-C-lowering medications, including statins, ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and inclisiran. Recently, angiopoietin-like protein 3 (ANGPTL3) inhibitors have emerged as a significant addition to lipid-lowering therapies, particularly for patients with challenging cases of hypercholesterolemia, such as those with homozygous familial hypercholesterolemia (HoFH). Thus, all these factors drive the ANGPTL3-lowering therapy market growth.
Furthermore, key players in the industry more focus on the research studies and key developments that would drive this global ANGPTL3-lowering therapy market growth. For instance, according to the NCBI research publication in November 2022, elevated levels of low-density lipoprotein cholesterol (LDL-C) and triglyceride-rich lipoproteins (TRLs) are significant contributors to the risk of developing atherosclerotic cardiovascular disease (ASCVD). The persistent difficulty in achieving recommended LDL-C targets, even with the maximum tolerated lipid-lowering therapies (LLT), has prompted the creation of innovative therapeutic agents, including angiopoietin-like 3 (ANGPTL3) inhibitors. All these factors demand the global ANGPTL3-lowering therapy market.
Moreover, the rising demand for emerging therapeutic innovations contributes to the global ANGPTL3-lowering therapy market expansion.
Availability of Limited Treatment Options
The limited availability of treatment options presents a significant challenge in the global ANGPTL3-lowering therapy market, particularly for patients suffering from conditions like homozygous familial hypercholesterolemia (HoFH).
As per Ultragenyx Pharmaceutical Inc. news in January 2024, Homozygous familial hypercholesterolemia (HoFH) is a severe form of inherited hypercholesterolemia that affects approximately 1 in 300,000 individuals worldwide and around 1,600 people in the European Union. This condition arises when a person inherits two copies of genes that cause familial hypercholesterolemia (FH) one from each parent resulting in dangerously high levels of low-density lipoprotein cholesterol (LDL-C), often exceeding 400 mg/dL. Individuals with HoFH face a heightened risk of developing premature atherosclerotic disease and experiencing cardiac events at an early age. All these limit the ANGPTL3-lowering therapy market growth.
HoFH is classified as an ultra-rare genetic disorder, affecting approximately 1,300 patients in the U.S. This small patient population inherently restricts the market for ANGPTL3 inhibitors, such as evinacumab. The rarity of HoFH can dissuade pharmaceutical companies from investing heavily in the development of additional therapies, resulting in a limited number of treatment options available to patients. The availability of limited treatment options will hinder the growth of the global ANGPTL3-lowering therapy market.
Currently, evinacumab is primarily utilized as an adjunct therapy alongside existing lipid-lowering treatments, including statins and PCSK9 inhibitors. Many patients with HoFH are already on multiple lipid-lowering medications, but the effectiveness of these combinations can vary significantly due to individual genetic differences. For instance, patients lacking functional LDL receptors may not respond to PCSK9 inhibitors, further constraining the therapeutic landscape. Thus, the above factors could be limiting the global ANGPTL3-lowering therapy market's potential growth.
Segment Analysis
The global ANGPTL3-lowering therapy market is segmented based on treatment type, application, and region.
Treatment Type:
The monoclonal antibodies segment is expected to dominate the global ANGPTL3-lowering therapy market share
The monoclonal antibodies segment holds a major portion of the global ANGPTL3-lowering therapy market share and is expected to continue to hold a significant portion of the global ANGPTL3-lowering therapy market share during the forecast period.
Evkeeza (evinacumab-dgnb) is a monoclonal antibody that specifically binds to and inhibits the function of angiopoietin-like protein 3 (ANGPTL3). ANGPTL3 is a protein that slows down the activity of certain enzymes responsible for breaking down fats in the body.
By blocking ANGPTL3, Evkeeza facilitates a faster breakdown of fats, which helps lower cholesterol levels. As per FDA approval in February 2021, the effectiveness and safety of Evkeeza were assessed in a double-blind, randomized, placebo-controlled trial lasting 24 weeks, which included 65 patients diagnosed with homozygous familial hypercholesterolemia (HoFH). In this study, 43 patients received a dosage of 15 mg/kg of Evkeeza every four weeks, while 22 patients were given a placebo. All participants were also undergoing treatment with other lipid-lowering therapies during the trial. All these factors demand the monoclonal antibodies segment in the ANGPTL3-lowering therapy market.
Furthermore, key players in the industry product launches & approvals, and research activities would propel this segment growth in the global angptl3-lowering therapy market. For instance, as per NCBI research publication in October 2023, Evinacumab (evinacumab-dgnb) received approval from the United States Food and Drug Administration (FDA) in 2021 as an adjunct therapy to other cholesterol-lowering treatments for patients aged 5 years and older diagnosed with homozygous familial hypercholesterolemia (HoFH).
HoFH is a rare genetic disorder that severely impacts lipid metabolism, leading to significantly elevated low-density lipoprotein cholesterol (LDL-C) levels of 300 mg/dL or higher, despite the use of standard oral therapies. This condition arises from diminished or absent LDL-receptor function, which is crucial for the clearance of LDL-C from the bloodstream. These factors have solidified the segment's position in the global ANGPTL3-lowering therapy market.
Geographical Analysis
North America is expected to hold a significant position in the global ANGPTL3-lowering therapy market share
North America holds a substantial position in the global ANGPTL3-lowering therapy market and is expected to hold most of the market share.
The rising prevalence of hyperlipidemia, particularly elevated levels of low-density lipoprotein cholesterol (LDL-C) and triglycerides, significantly drives the ANGPTL3-lowering therapy market. Hyperlipidemia is a critical risk factor for atherosclerotic cardiovascular disease (ASCVD), which remains the leading cause of death in North America. As awareness of cardiovascular risks increases, the demand for effective treatments to manage these conditions becomes more urgent.
Furthermore, in this region, a major number of key players' presence, well-advanced healthcare infrastructure, government initiatives & regulatory support, investments, and product launches & approvals would propel the global ANGPTL3-lowering therapy market growth. For instance, in March 2023, Regeneron Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has extended the approval of Evkeeza (evinacumab-dgnb) to include its use as an adjunct therapy for children aged 5 to 11 years diagnosed with homozygous familial hypercholesterolemia (HoFH). This approval marks Evkeeza as the first angiopoietin-like protein 3 (ANGPTL3) inhibitor specifically indicated for this age group, aimed at controlling dangerously high levels of low-density lipoprotein cholesterol (LDL-C) associated with HoFH. Thus, the above factors are consolidating the region's position as a dominant force in the global ANGPTL3-lowering therapy market.
Asia Pacific is growing at the fastest pace in the global ANGPTL3-lowering therapy market
Asia Pacific holds the fastest pace in the global ANGPTL3-lowering therapy market and is expected to hold most of the market share.
The rising incidence of hyperlipidemia is a significant factor driving the ANGPTL3-lowering therapy market. Heart Foundation data in January 2024, more than 2 in 5 (42%) of Australian adults are affected by high cholesterol, with the condition being most prevalent among individuals aged 55 to 64 years. Despite this significant statistic, only 7% of adults identify high cholesterol as a major risk factor for heart disease. This discrepancy indicates a concerning lack of awareness regarding the dangers associated with elevated cholesterol levels and their role in cardiovascular health.
The limited awareness of high cholesterol as a significant risk factor for atherosclerotic cardiovascular disease (ASCVD) among Australians highlights the urgent need for enhanced public education and outreach efforts. This gap in understanding offers a valuable opportunity for the ANGPTL3-lowering therapy market to grow by addressing the unmet clinical needs, particularly among high-risk populations. By promoting effective treatments such as evinacumab and increasing awareness about the importance of managing cholesterol levels, stakeholders can play a crucial role in improving cardiovascular health outcomes throughout Australia.
Furthermore, key players in the region introducing emerging RNA interference therapies, approval of innovative therapies, and growing awareness and screening initiatives are driving the ANGPTL3-lowering therapy market. For instance, according to the NCBI research publication in August 2023, innovative RNA interference therapies targeting ANGPTL3 are beginning to make their way into the Australian market. For instance, ARO-ANG3 has demonstrated the ability to reduce ANGPTL3 levels by 45% to 78%, along with significant decreases in triglycerides, with median reductions ranging from 34% to 54%. These emerging therapies provide additional options for effectively managing dyslipidemia.
Thus, the above factors are consolidating the region's position as the fastest-growing force in the global ANGPTL3-lowering therapy market.
Competitive Landscape & Emerging Players
The major global players & emerging players in the ANGPTL3-lowering therapy market include Regeneron Pharmaceuticals, Inc., Arrowhead Pharmaceuticals Inc., Amgen Inc., Ionis Pharmaceuticals, Inc. (Akcea Therapeutics), Silence Therapeutics, Eli Lilly and Company, Novartis AG, Verve Therapeutics, Inc., and CRISPR Therapeutics. Among others.
Key Developments
• In January 2024, Ultragenyx Pharmaceutical Inc. announced that the National Institute for Health and Care Excellence (NICE) has released a final draft guidance recommending Evkeeza (evinacumab) for use within NHS England. Evkeeza is advised as an adjunct to diet and other therapies that lower low-density lipoprotein cholesterol (LDL-C) for treating adults and adolescents aged 12 years and older diagnosed with homozygous familial hypercholesterolemia (HoFH). This marks Evkeeza as the first treatment targeting angiopoietin-like protein 3 (ANGPTL3) to be indicated for this rare and debilitating condition.
Why Purchase the Report?
• To visualize the global ANGPTL3-lowering therapy market segmentation based on treatment type, application, and region and understand key commercial assets and players.
• Identify commercial opportunities by analyzing trends and co-development.
• Excel data sheet with numerous data points of theANGPTL3-lowering therapy market with all segments.
• PDF report consists of a comprehensive analysis after exhaustive qualitative interviews and an in-depth study.
• Product mapping is available in excel consisting of key products of all the major players.
The global ANGPTL3-lowering therapy market report would provide approximately 51 tables, 45 figures, and 184 pages.
Target Audience 2023
• Manufacturers/ Buyers
• Industry Investors/Investment Bankers
• Research Professionals
• Emerging Companies
1. Methodology and Scope
1.1. Research Methodology
1.2. Research Objective and Scope of the Report
2. Definition and Overview
3. Executive Summary
3.1. Snippet by Treatment Type
3.2. Snippet by Application
3.3. Snippet by Region
4. Dynamics
4.1. Impacting Factors
4.1.1. Drivers
4.1.1.1. Rising Prevalence of Hyperlipidemia and Cardiovascular Diseases (CVD)
4.1.1.2. XX
4.1.2. Restraints
4.1.2.1. Availability of Limited Treatment Options
4.1.3. Opportunity
4.1.4. Impact Analysis
5. Industry Analysis
5.1. Porter’s Five Force Analysis
5.2. Supply Chain Analysis
5.3. Pricing Analysis
5.4. Regulatory Analysis
6. By Treatment Type
6.1. Introduction
6.1.1. Analysis and Y-o-Y Growth Analysis (%), By Treatment Type
6.1.2. Market Attractiveness Index, By Treatment Type
6.2. Monoclonal Antibodies *
6.2.1. Introduction
6.2.2. Market Size Analysis and Y-o-Y Growth Analysis (%)
6.3. Antisense Oligonucleotides (ASOs)
6.4. RNA Interference (RNAi) Therapies (siRNA)
6.5. CRISPR-Based Gene Editing
6.6. Others
7. By Application
7.1. Introduction
7.1.1. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
7.1.2. Market Attractiveness Index, By Application
7.2. Familial Hypercholesterolemia (FH) *
7.2.1. Introduction
7.2.2. Market Size Analysis and Y-o-Y Growth Analysis (%)
7.3. Refractory Hyperlipidemia
7.4. Mixed Dyslipidemia
7.5. Cardiovascular Diseases
7.6. Others
8. By Region
8.1. Introduction
8.1.1. Market Size Analysis and Y-o-Y Growth Analysis (%), By Region
8.1.2. Market Attractiveness Index, By Region
8.2. North America
8.2.1. Introduction
8.2.2. Key Region-Specific Dynamics
8.2.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Treatment Type
8.2.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
8.2.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
8.2.5.1. U.S.
8.2.5.2. Canada
8.2.5.3. Mexico
8.3. Europe
8.3.1. Introduction
8.3.2. Key Region-Specific Dynamics
8.3.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Treatment Type
8.3.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
8.3.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
8.3.5.1. Germany
8.3.5.2. U.K.
8.3.5.3. France
8.3.5.4. Spain
8.3.5.5. Italy
8.3.5.6. Rest of Europe
8.4. South America
8.4.1. Introduction
8.4.2. Key Region-Specific Dynamics
8.4.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Treatment Type
8.4.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
8.4.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
8.4.5.1. Brazil
8.4.5.2. Argentina
8.4.5.3. Rest of South America
8.5. Asia-Pacific
8.5.1. Introduction
8.5.2. Key Region-Specific Dynamics
8.5.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Treatment Type
8.5.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
8.5.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
8.5.5.1. China
8.5.5.2. India
8.5.5.3. Japan
8.5.5.4. South Korea
8.5.5.5. Rest of Asia-Pacific
8.6. Middle East and Africa
8.6.1. Introduction
8.6.2. Key Region-Specific Dynamics
8.6.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Treatment Type
8.6.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
9. Competitive Landscape
9.1. Competitive Scenario
9.2. Market Positioning/Share Analysis
9.3. Mergers and Acquisitions Analysis
10. Company Profiles
10.1. Regeneron Pharmaceuticals, Inc.*
10.1.1. Company Overview
10.1.2. Product Portfolio and Description
10.1.3. Financial Overview
10.1.4. Key Developments
10.2. Arrowhead Pharmaceuticals Inc.
10.3. Amgen Inc.
10.4. Ionis Pharmaceuticals, Inc. (Akcea Therapeutics)
10.5. Silence Therapeutics
10.6. Eli Lilly and Company
10.7. Novartis AG
10.8. Verve Therapeutics, Inc.
10.9. CRISPR Therapeutics.
LIST NOT EXHAUSTIVE
11. Appendix
11.1. About Us and Services
11.2. Contact Us
*** ANGPTL3低下療法の世界市場に関するよくある質問(FAQ) ***
・ANGPTL3低下療法の世界市場規模は?
→DataM Intelligence社は2023年のANGPTL3低下療法の世界市場規模を7730万米ドルと推定しています。
・ANGPTL3低下療法の世界市場予測は?
→DataM Intelligence社は2031年のANGPTL3低下療法の世界市場規模を7億8521万米ドルと予測しています。
・ANGPTL3低下療法市場の成長率は?
→DataM Intelligence社はANGPTL3低下療法の世界市場が2024年~2031年に年平均30.9%成長すると展望しています。
・世界のANGPTL3低下療法市場における主要プレイヤーは?
→「Regeneron Pharmaceuticals, Inc.、Arrowhead Pharmaceuticals Inc.、Amgen Inc.、Ionis Pharmaceuticals, Inc.(Akcea Therapeutics)、Silence Therapeutics、Eli Lilly and Company、Novartis AG、Verve Therapeutics, Inc.、CRISPR Therapeuticsなど ...」をANGPTL3低下療法市場のグローバル主要プレイヤーとして判断しています。
※上記FAQの市場規模、市場予測、成長率、主要企業に関する情報は本レポートの概要を作成した時点での情報であり、最終レポートの情報と少し異なる場合があります。
*** 免責事項 ***
https://www.globalresearch.co.jp/disclaimer/











