1. 調査方法および範囲
1.1. 調査方法
1.2. 調査目的およびレポートの範囲
2. 定義および概要
3. エグゼクティブサマリー
3.1. 製品種類別抜粋
3.2. 材料種類別抜粋
3.3. 用途別抜粋
3.4. エンドユーザー別抜粋
3.5. 地域別
4. ダイナミクス
4.1. 影響因子
4.1.1. 推進要因
4.1.1.1. ヘルニアの有病率の増加
4.1.1.2. 外科用メッシュ設計における技術革新の進展
4.1.2. 抑制要因
4.1.2.1. 合併症と安全性への懸念
4.1.3. 機会
4.1.4. 影響分析
5. 産業分析
5.1. ポーターのファイブフォース分析
5.2. サプライチェーン分析
5.3. 価格分析
5.4. 特許分析
5.5. 規制分析
5.6. SWOT分析
5.7. 未充足ニーズ
6. 製品の種類別
6.1. はじめに
6.1.1. 市場規模分析および前年比成長率(%)製品の種類別
6.1.2. 市場魅力度指数、製品の種類別
6.2. 吸収性外科用メッシュ*
6.2.1. はじめに
6.2.2. 市場規模分析および前年比成長率(%)
6.3. 非吸収性外科用メッシュ
6.4. 生体吸収性メッシュ
6.5. 合成メッシュ
7. 素材の種類別
7.1. はじめに
7.1.1. 素材の種類別市場規模分析および前年比成長率(%)
7.1.2. 素材の種類別市場魅力度指数
7.2. ポリプロピレン*
7.2.1. はじめに
7.2.2. 市場規模分析および前年比成長率分析(%)
7.3. ポリエステル
7.4. ポリテトラフルオロエチレン(PTFE)
7.5. その他
8. 用途別
8.1. はじめに
8.1.1. 市場規模分析および前年比成長率分析(%)、用途別
8.1.2. 用途別市場魅力度指数
8.2. ヘルニア修復術*
8.2.1. はじめに
8.2.2. 市場規模分析および前年比成長率分析(%)
8.3. 骨盤臓器脱修復術
8.4. 腹圧性尿失禁
8.5. その他
9. エンドユーザー別
9.1. はじめに
9.1.1. エンドユーザー別市場規模分析および前年比成長率(%)
9.1.2. エンドユーザー別市場魅力度指数
9.2. 病院*
9.2.1. はじめに
9.2.2. 市場規模分析および前年比成長率(%)
9.3. 専門クリニック
9.4. 外来外科手術センター
9.5. その他
10. 地域別
10.1. はじめに
10.1.1. 地域別市場規模分析および前年比成長率分析(%)
10.1.2. 地域別市場魅力度指数
10.2. 北米
10.2.1. はじめに
10.2.2. 主要地域別の動向
10.2.3. 市場規模および前年比成長率(%)製品種類別
10.2.4. 市場規模および前年比成長率(%)材料種類別
10.2.5. 市場規模および前年比成長率(%)用途別
10.2.6. エンドユーザー別市場規模分析および前年比成長率分析(%)
10.2.7. 国別市場規模分析および前年比成長率分析(%)
10.2.7.1. 米国
10.2.7.2. カナダ
10.2.7.3. メキシコ
10.3. ヨーロッパ
10.3.1. はじめに
10.3.2. 主要地域別の動向
10.3.3. 製品タイプ別市場規模分析および前年比成長率(%)
10.3.4. 材料タイプ別市場規模分析および前年比成長率(%)
10.3.5. 用途別市場規模分析および前年比成長率(%)
10.3.6. エンドユーザー別市場規模分析および前年比成長率(%)
10.3.7. 国別市場規模分析および前年比成長率(%)
10.3.7.1. ドイツ
10.3.7.2. 英国
10.3.7.3. フランス
10.3.7.4. スペイン
10.3.7.5. イタリア
10.3.7.6. ヨーロッパのその他地域
10.4. 南アメリカ
10.4.1. はじめに
10.4.2. 主要地域別の動向
10.4.3. 製品種類別市場規模分析および前年比成長率分析(%)
10.4.4. 市場規模分析および前年比成長率(%)、材料の種類別
10.4.5. 市場規模分析および前年比成長率(%)、用途別
10.4.6. 市場規模分析および前年比成長率(%)、エンドユーザー別
10.4.7. 市場規模分析および前年比成長率(%)、国別
10.4.7.1. ブラジル
10.4.7.2. アルゼンチン
10.4.7.3. 南米その他
10.5. アジア太平洋地域
10.5.1. はじめに
10.5.2. 地域特有の主な動向
10.5.3. 市場規模分析および前年比成長率分析(%)、製品種類別
10.5.4. 市場規模分析および前年比成長率(%)、材料の種類別
10.5.5. 市場規模分析および前年比成長率(%)、用途別
10.5.6. 市場規模分析および前年比成長率(%)、エンドユーザー別
10.5.7. 市場規模分析および前年比成長率(%)、国別
10.5.7.1. 中国
10.5.7.2. インド
10.5.7.3. 日本
10.5.7.4. 韓国
10.5.7.5. アジア太平洋地域その他
10.6. 中東およびアフリカ
10.6.1. はじめに
10.6.2. 主要地域特有の動向
10.6.3. 製品種類別市場規模分析および前年比成長率(%)
10.6.4. 材料種類別市場規模分析および前年比成長率(%)
10.6.5. 用途別市場規模分析および前年比成長率(%)
10.6.6. エンドユーザー別市場規模分析および前年比成長率(%)
10.6.7. エンドユーザー別市場規模分析および前年比成長率分析(%)
11. 競合状況
11.1. 競合シナリオ
11.2. 市場ポジショニング/シェア分析
11.3. 合併・買収分析
12. 企業プロフィール
Johnson & Johnson
B. Braun SE
Medtronic plc
Becton, Dickinson and Company
CITEC
W. L. Gore & Associates, Inc.
Medcity Surgicals
Dolphin Sutures
Herniamesh S.r.l.
Advin Health Care
(リストは網羅的なものではありません)
13. 付録
13.1. 当社およびサービスについて
13.2. お問い合わせ
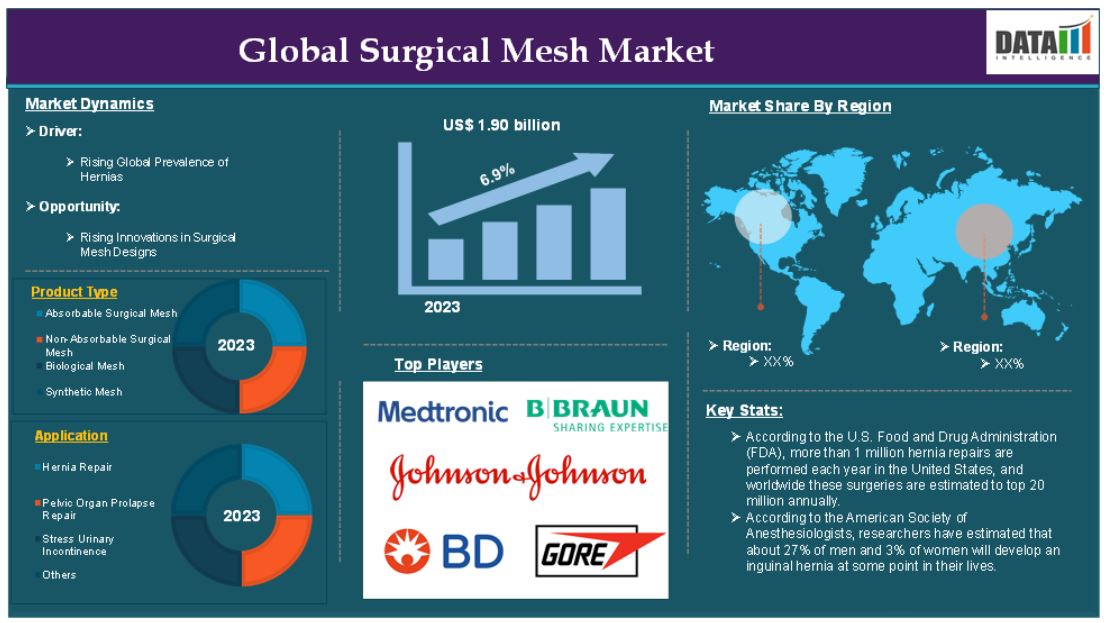
The global surgical mesh market reached US$ 1.90 billion in 2023 and is expected to reach US$ 3.22 billion by 2031, growing at a CAGR of 6.9% during the forecast period 2024-2031.
Surgical mesh is a medical device used in surgical procedures to provide additional support to weakened or damaged tissue. It is typically a flexible, sheet-like structure made from synthetic or biological materials and is designed to reinforce and stabilize tissues during healing. Surgical meshes are widely used in various surgical applications, including hernia repair, pelvic organ prolapse (POP) repair, stress urinary incontinence (SUI) treatment and other reconstructive surgeries. Meshes are available in various structures, such as monofilament, multifilament and composite meshes, to cater to specific surgical needs.
The surgical mesh market has witnessed substantial demand over the years owing to the rising hernia repairs, especially. For instance, according to the U.S. Food and Drug Administration (FDA), more than 1 million hernia repairs are performed each year in the United States, and worldwide these surgeries are estimated to top 20 million annually, these repairs involve the use of surgical mesh, making it the standard of care for hernia surgery, contributing to the increasing demand for surgical meshes.
Market Dynamics: Drivers & Restraints
Rising prevalence of hernias
The rising prevalence of hernias is significantly driving the surgical mesh market, as hernia repairs are one of the most common surgical applications for mesh implants. Hernia is a condition in which an organ or tissue bulges through a weak spot in the surrounding muscle or connective tissue. For instance, aLLSurgical mesh is used to reinforce the weakened area, ensuring better recovery outcomes and reducing the risk of recurrence.
Additionally, hernia repairs are among the most common types of surgeries performed worldwide. According to the U.S. Food and Drug Administration (FDA), annually, about 20 million hernia repairs are conducted globally. This high volume directly drives demand for surgical meshes used in these procedures, especially for inguinal, umbilical, and ventral hernias.
Surgical mesh is considered the gold standard for hernia repair due to its ability to provide long-term reinforcement to the tissue. 80% of all hernia repairs involve the use of mesh, as it helps prevent recurrence and speeds up recovery. This preference for mesh implants in hernia surgeries significantly contributes to the growth of the surgical mesh market.
Complications and safety concerns
Complications and safety concerns related to surgical mesh are expected to hamper the growth of the surgical mesh market. These concerns revolve around issues such as mesh migration, infection, erosion, chronic pain and long-term health complications, all of which can lead to negative patient outcomes and increased healthcare costs. The growing number of adverse events and safety concerns surrounding surgical meshes has prompted regulatory scrutiny and legal challenges, which can slow the adoption of mesh products.
Mesh erosion occurs when the mesh material degrades or moves from its original position, causing it to become embedded in nearby tissues or organs. This can lead to severe complications such as infection, chronic pain, and tissue damage. Similarly, mesh migration refers to the movement of the mesh from its intended placement, which can also cause internal damage and necessitate corrective surgery.
For instance, studies suggest that 5% to 20% of hernia operations result in mesh failure. According to a study in the British Medical Journal, the rate could be between 12% and 30%. These complications may require revision surgeries, which can increase both healthcare costs and patient morbidity.
Segment Analysis
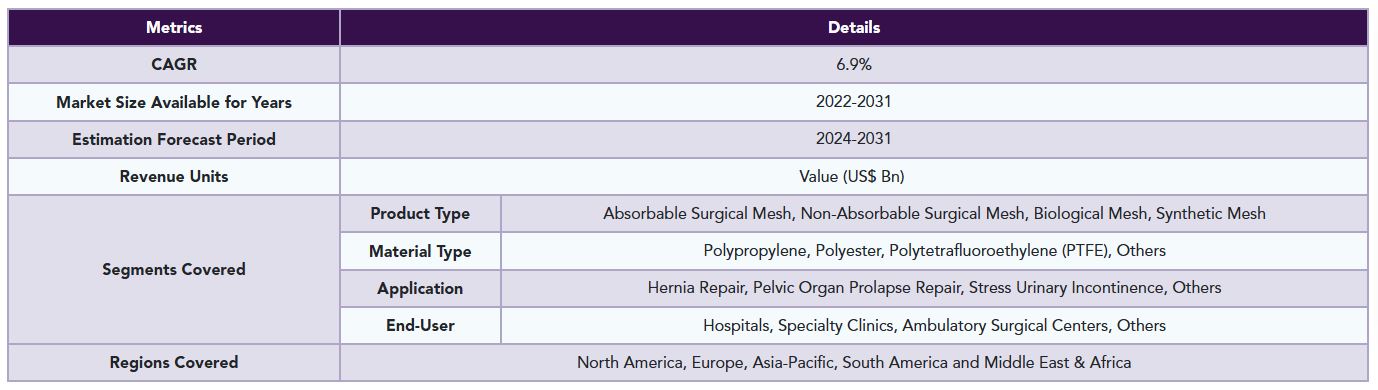
The global surgical mesh market is segmented based on product type, material type, application, end-user and region.
Application:
The hernia repair segment is expected to dominate the global surgical mesh market share
The hernia repair segment holds a major portion of the surgical mesh market share and is expected to continue to hold a significant portion of the market share over the forecast period due to the established use of mesh in hernia repair surgeries and the superior outcomes provided by mesh-based procedures compared to traditional non-mesh surgeries. Hernia repair surgeries are the gold standard for reinforcing the weakened abdominal walls and preventing hernia recurrence. Thus, the market players are focusing on surgical mesh development due to its wide advantages.
For instance, in November 2024, Deep Blue Medical expanded its success of T-Line Hernia Mesh, a surgical product that helps prevent the recurrence of hernias after abdominal hernia repair surgery. Although the T-Line Mesh made its clinical debut just three years ago, it is already being used by about 45 clinical sites in the United States and over 8,000 of the novel extensions and lockstitches of the mesh have been implanted to date with no adverse events.
The shift towards minimally invasive hernia repair surgeries, such as laparoscopic and robot-assisted surgery, has contributed to the dominance of the hernia repair segment. Surgical meshes are ideal for use in these procedures because they are lightweight, flexible and can be easily inserted through small incisions.
For instance, laparoscopic hernia repair is becoming the preferred technique for many surgeons due to its lower complication rates and quicker recovery. According to the Global Hernia Society, the laparoscopic approach accounts for about 60-70% of hernia surgeries in developed countries, driving the demand for surgical meshes tailored for these procedures.
With the increasing awareness of hernias and the effectiveness of mesh-based surgeries, more patients are seeking treatment, contributing to the growing demand for hernia repairs using mesh. Additionally, improvements in healthcare access in emerging economies have increased the number of hernia surgeries being performed in those regions.
For instance, in June 2021, BG Medical., the manufacturer and distributor of the 21st Century SURGIMESH Platform honored Hernia Awareness Month by partnering with CQInsights to pioneer a new approach that delivers better outcomes for patients, with a larger mission to share sustainable solutions that improve healthcare for everyone.
North America is expected to hold a significant position in the global surgical mesh market
North America represents the largest share of the global surgical mesh market in terms of both revenue generation and market size. The U.S., in particular, is the largest market within the region, driven by a combination of high demand for surgeries, advanced healthcare technologies, and a well-established healthcare system.
North America, particularly the United States, has a high incidence of hernias due to factors such as an aging population, high obesity rates, and sedentary lifestyles. This has led to a large number of hernia repairs, most of which involve surgical mesh. For instance, according to Healthcare Cost and Utilization Project data and the US Food and Drug Administration, an estimated 611,000 ventral and 1 million inguinal hernia repairs are performed each year in the US.
North America has high levels of healthcare awareness and access, which leads to an increased number of surgeries involving surgical mesh. Patients are more likely to seek treatment for conditions like hernias, knowing that advanced treatments, such as mesh implants, are available. Thus, the market players are focusing on launching the surgical mesh with technological advancements.
For instance, in April 2024, ELA Bio commercially launched the OviTex Inguinal Hernia Repair (IHR) mesh in the US. The Pennsylvania-based firm has specifically designed the robotic-compatible OviTex IHR surgical mesh for use in laparoscopic and inguinal hernia repair. It comes in a three or four layer anatomically shaped device or a three-layer rectangular device.
Asia Pacific is growing at the fastest pace in the surgical mesh market
The Asia Pacific region is experiencing the fastest growth in the surgical mesh market. The rising rates of obesity in countries like China and India, combined with an increasing number of abdominal hernia cases, are driving the demand for hernia repair surgeries. Obesity is a key risk factor for hernias, and with lifestyle changes, these conditions are becoming more prevalent in the Asia Pacific region, creating a significant market opportunity for surgical meshes.
For instance, according to the World Health Organization (WHO), the prevalence of obesity in Asia is rising rapidly. According to the Chinese BMI classification for overweight and obesity, 34.8% of the study population were overweight and 14.1% were obese. This growing rate of obesity significantly contributes to the increasing number of hernia cases and surgeries requiring mesh.
The Asia Pacific region has witnessed significant investments in healthcare infrastructure, particularly in countries like India, China and South Korea, where hospitals, surgical centers and medical device manufacturers are modernizing and expanding. This has made advanced surgical treatments, including mesh-based procedures, more accessible to the growing population.
For instance, in October 2024, Park Hospital, a multi-specialty healthcare provider performed a complex robotic-assisted surgery to save a 45-year-old man from a complicated diaphragmatic hernia. Robotic-assisted surgery allowed to safely reduce the herniated organs back into the abdominal cavity and repair the diaphragm with a large mesh, ensuring a stable closure. This increases the demand for surgical mesh in the region.
Competitive Landscape
The major global players in the surgical mesh market include Johnson & Johnson, B. Braun SE, Medtronic plc, Becton, Dickinson and Company, CITEC, W. L. Gore & Associates, Inc., Medcity Surgicals, Dolphin Sutures, Herniamesh S.r.l., Advin Health Care and among others.
Why Purchase the Report?
• Pipeline & Innovations: Insights into clinical trials, product pipelines, and upcoming advancements.
• Market Positioning: Analysis of product performance and growth potential for optimized strategies.
• Real-World Evidence: Integration of patient feedback for enhanced product outcomes.
• Physician Preferences: Insights into healthcare provider behaviors and adoption strategies.
• Regulatory & Market Updates: Coverage of recent regulations, policies, and emerging technologies.
• Competitive Insights: Analysis of market share, competitor strategies, and new entrants.
• Pricing & Market Access: Review of pricing models, reimbursement trends, and access strategies.
• Market Expansion: Strategies for entering new markets and forming partnerships.
• Regional Opportunities: Identification of high-growth regions and investment prospects.
• Supply Chain Optimization: Assessment of risks and distribution strategies.
• Sustainability & Regulation: Focus on eco-friendly practices and regulatory changes.
• Post-Market Surveillance: Enhanced safety and access through post-market data.
• Value-Based Pricing: Insights into pharmacoeconomics and data-driven R&D decisions.
The global surgical mesh market report delivers a detailed analysis with 70 key tables, more than 66 visually impactful figures, and 176 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2023
• Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
• Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
• Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
• Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
• Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
• Supply Chain: Distribution and Supply Chain Managers.
• Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
• Academic & Research: Academic Institutions.
Table of Contents
1. Methodology and Scope
1.1. Research Methodology
1.2. Research Objective and Scope of the Report
2. Definition and Overview
3. Executive Summary
3.1. Snippet by Product Type
3.2. Snippet by Material Type
3.3. Snippet by Application
3.4. Snippet by End-User
3.5. Snippet by Region
4. Dynamics
4.1. Impacting Factors
4.1.1. Drivers
4.1.1.1. Rising Prevalence of Hernias
4.1.1.2. Rising Innovations in Surgical Mesh Designs
4.1.2. Restraints
4.1.2.1. Complications and Safety Concerns
4.1.3. Opportunity
4.1.4. Impact Analysis
5. Industry Analysis
5.1. Porter’s Five Force Analysis
5.2. Supply Chain Analysis
5.3. Pricing Analysis
5.4. Patent Analysis
5.5. Regulatory Analysis
5.6. SWOT Analysis
5.7. Unmet Needs
6. By Product Type
6.1. Introduction
6.1.1. Market Size Analysis and Y-o-Y Growth Analysis (%), By Product Type
6.1.2. Market Attractiveness Index, By Product Type
6.2. Absorbable Surgical Mesh*
6.2.1. Introduction
6.2.2. Market Size Analysis and Y-o-Y Growth Analysis (%)
6.3. Non-Absorbable Surgical Mesh
6.4. Biological Mesh
6.5. Synthetic Mesh
7. By Material Type
7.1. Introduction
7.1.1. Market Size Analysis and Y-o-Y Growth Analysis (%), By Material Type
7.1.2. Market Attractiveness Index, By Material Type
7.2. Polypropylene*
7.2.1. Introduction
7.2.2. Market Size Analysis and Y-o-Y Growth Analysis (%)
7.3. Polyester
7.4. Polytetrafluoroethylene (PTFE)
7.5. Others
8. By Application
8.1. Introduction
8.1.1. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
8.1.2. Market Attractiveness Index, By Application
8.2. Hernia Repair*
8.2.1. Introduction
8.2.2. Market Size Analysis and Y-o-Y Growth Analysis (%)
8.3. Pelvic Organ Prolapse Repair
8.4. Stress Urinary Incontinence
8.5. Others
9. By End-User
9.1. Introduction
9.1.1. Market Size Analysis and Y-o-Y Growth Analysis (%), By End-User
9.1.2. Market Attractiveness Index, By End-User
9.2. Hospitals*
9.2.1. Introduction
9.2.2. Market Size Analysis and Y-o-Y Growth Analysis (%)
9.3. Specialty Clinics
9.4. Ambulatory Surgical Centers
9.5. Others
10. By Region
10.1. Introduction
10.1.1. Market Size Analysis and Y-o-Y Growth Analysis (%), By Region
10.1.2. Market Attractiveness Index, By Region
10.2. North America
10.2.1. Introduction
10.2.2. Key Region-Specific Dynamics
10.2.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Product Type
10.2.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Material Type
10.2.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
10.2.6. Market Size Analysis and Y-o-Y Growth Analysis (%), By End-User
10.2.7. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
10.2.7.1. U.S.
10.2.7.2. Canada
10.2.7.3. Mexico
10.3. Europe
10.3.1. Introduction
10.3.2. Key Region-Specific Dynamics
10.3.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Product Type
10.3.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Material Type
10.3.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
10.3.6. Market Size Analysis and Y-o-Y Growth Analysis (%), By End-User
10.3.7. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
10.3.7.1. Germany
10.3.7.2. U.K.
10.3.7.3. France
10.3.7.4. Spain
10.3.7.5. Italy
10.3.7.6. Rest of Europe
10.4. South America
10.4.1. Introduction
10.4.2. Key Region-Specific Dynamics
10.4.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Product Type
10.4.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Material Type
10.4.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
10.4.6. Market Size Analysis and Y-o-Y Growth Analysis (%), By End-User
10.4.7. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
10.4.7.1. Brazil
10.4.7.2. Argentina
10.4.7.3. Rest of South America
10.5. Asia-Pacific
10.5.1. Introduction
10.5.2. Key Region-Specific Dynamics
10.5.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Product Type
10.5.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Material Type
10.5.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
10.5.6. Market Size Analysis and Y-o-Y Growth Analysis (%), By End-User
10.5.7. Market Size Analysis and Y-o-Y Growth Analysis (%), By Country
10.5.7.1. China
10.5.7.2. India
10.5.7.3. Japan
10.5.7.4. South Korea
10.5.7.5. Rest of Asia-Pacific
10.6. Middle East and Africa
10.6.1. Introduction
10.6.2. Key Region-Specific Dynamics
10.6.3. Market Size Analysis and Y-o-Y Growth Analysis (%), By Product Type
10.6.4. Market Size Analysis and Y-o-Y Growth Analysis (%), By Material Type
10.6.5. Market Size Analysis and Y-o-Y Growth Analysis (%), By Application
10.6.6. Market Size Analysis and Y-o-Y Growth Analysis (%), By End-User
10.6.7. Market Size Analysis and Y-o-Y Growth Analysis (%), By End-User
11. Competitive Landscape
11.1. Competitive Scenario
11.2. Market Positioning/Share Analysis
11.3. Mergers and Acquisitions Analysis
12. Company Profiles
12.1. Johnson & Johnson*
12.1.1. Company Overview
12.1.2. Product Portfolio and Description
12.1.3. Financial Overview
12.1.4. Key Developments
12.2. B. Braun SE
12.3. Medtronic plc
12.4. Becton, Dickinson and Company
12.5. CITEC
12.6. W. L. Gore & Associates, Inc.
12.7. Medcity Surgicals
12.8. Dolphin Sutures
12.9. Herniamesh S.r.l.
12.10. Advin Health Care (LIST NOT EXHAUSTIVE )
13. Appendix
13.1. About Us and Services
13.2. Contact Us
*** 外科用メッシュの世界市場に関するよくある質問(FAQ) ***
・外科用メッシュの世界市場規模は?
→DataM Intelligence社は2023年の外科用メッシュの世界市場規模を19億米ドルと推定しています。
・外科用メッシュの世界市場予測は?
→DataM Intelligence社は2031年の外科用メッシュの世界市場規模を32億2000万米ドルと予測しています。
・外科用メッシュ市場の成長率は?
→DataM Intelligence社は外科用メッシュの世界市場が2024年~2031年に年平均6.9%成長すると展望しています。
・世界の外科用メッシュ市場における主要プレイヤーは?
→「Johnson & Johnson、B. Braun SE、Medtronic plc、Becton, Dickinson and Company、CITEC、W. L. Gore & Associates, Inc.、Medcity Surgicals、Dolphin Sutures、Herniamesh S.r.l.、Advin Health Careなど ...」を外科用メッシュ市場のグローバル主要プレイヤーとして判断しています。
※上記FAQの市場規模、市場予測、成長率、主要企業に関する情報は本レポートの概要を作成した時点での情報であり、最終レポートの情報と少し異なる場合があります。
*** 免責事項 ***
https://www.globalresearch.co.jp/disclaimer/











